New FDA Guidelines Expand Blood Donor Eligibility
Amy Barczy
| 2 min read
Amy Barczy is a former brand journalist who authored...
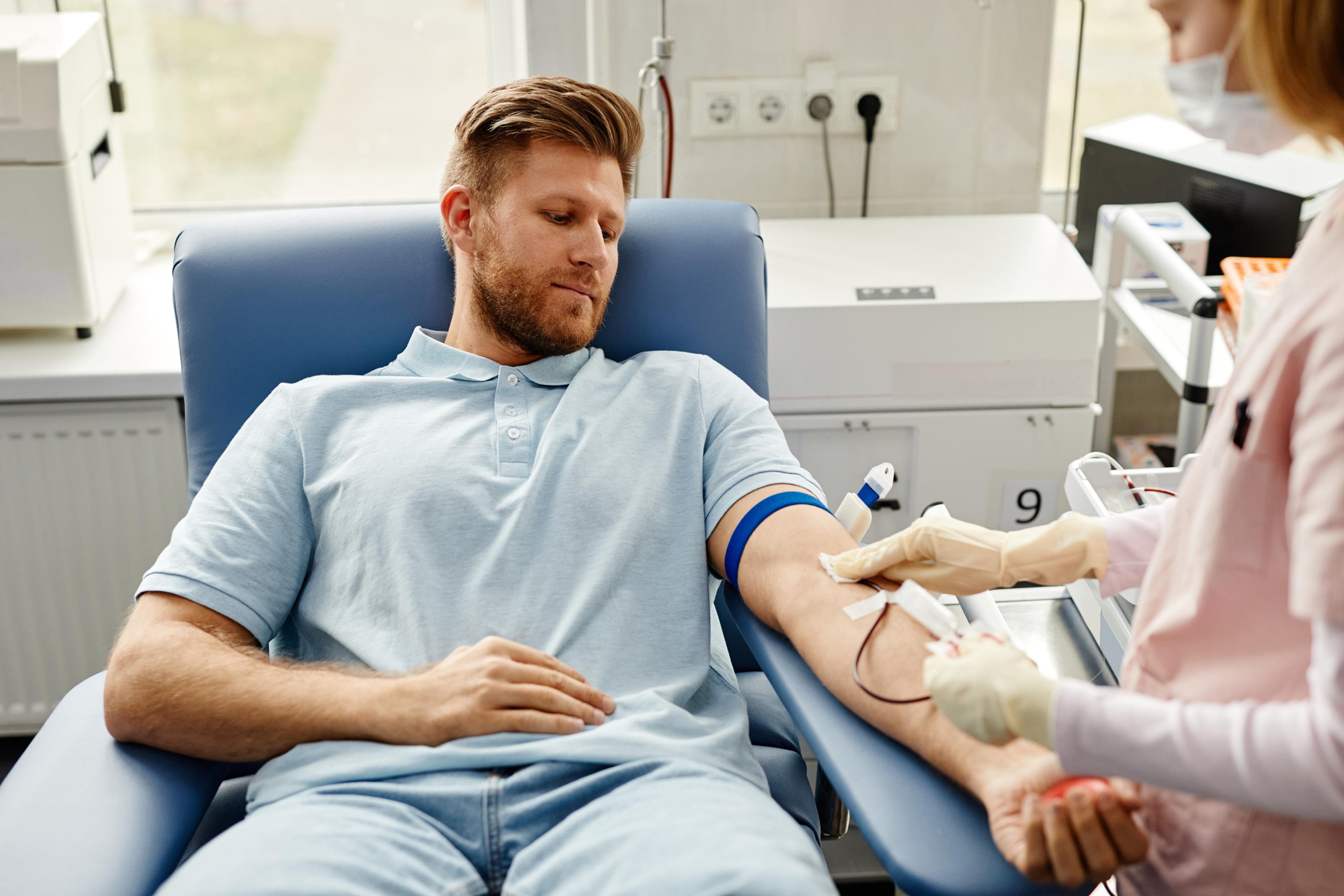
More gay and bisexual individuals will be able to donate blood, under new guidelines issued this month by the U.S. Food and Drug Administration.
The new guidelines use a set of individual, risk-based questions to screen donors, regardless of sexual orientation, sex or gender. The questions are intended to screen for risk of transfusion-transmitted HIV. These guidelines align with scientific evidence and are similar to international policies, including Canada and the U.K.
These new guidelines open the door for most gay men in monogamous relationships to become blood donors. Previously, there were specific screening questions and time-based deferrals just for gay men: men were asked to abstain from having sex with another man for three months before donating blood.
“The FDA has worked diligently to evaluate our policies and ensure we had the scientific evidence to support individual risk assessment for donor eligibility while maintaining appropriate safeguards to protect recipients of blood products. The implementation of these recommendations will represent a significant milestone for the agency and the LGBTQI+ community,” said Peter Marks, M.D., PhD., director of the FDA’s Center for Biologics Evaluation and Research, in a news release.
Now, all individuals seeking to become donors who have had anal sex with a new partner – or more than one sexual partner – in the past three months, will not be allowed to donate at that time. Individuals taking medications to treat or prevent HIV infection will also be deferred from donating under the new guidelines.
Leading blood donation organizations, including the American Red Cross, America’s Blood Centers and the Association for the Advancement of Blood & Biotherapies supported the FDA’s rule change expanding donor eligibility.
These new eligibility guidelines are being implemented at blood collection centers across the U.S.
The old FDA guidelines for men who have sex with men are rooted in the precautions taken to prevent the spread of HIV during the beginning of the AIDS epidemic. Gay and bisexual men were kept from donating blood at first – rules that were relaxed over time; but different standards still applied until now.
After blood is donated, blood banks test donations for a number of diseases, including HIV, before the blood can be used to help patients in need.
Each year, an estimated 6.8 million people in the U.S. donate blood, according to the American Red Cross. One person’s donation can save up to three lives. The demand for blood donations is constant. Donating blood only takes about an hour, yet only 3% of the eligible donor population gives each year.